LONGVIDA® primed for Sports Nutrition: Batch verified for Sports banned Substances + Vascular endothelial Health Study
MAY 2017 –Longvida® Optimized Curcumin passed rigorous testing for banned substances for professional athletes. The batch tested below detection limits for a screen of over 200 compounds. This batch-specific certification, Certified for Sports by Korva Labs, is announced and available to customers for purchase moving forward. Verdure is excited to expand the pipeline of quality and assurance parameters with another available certification.
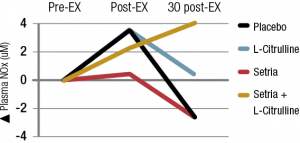
Representatives will be available to answer your questions in person at Vitafoods booth H2O where they are excited to talk about the independent NIH funded study in Aging; Curcumin Nitric oxide bioavailability is important for healthy joints as nitric oxide is known to be a key mediator of vasodilation, inflammation, and pain perception. After 12 weeks of supplementation, forearm blood flow increased 37% for those taking Longvida® (P=0.03), whereas no change was seen in the placebo group (P=0.2). Furthermore, the scientists go on to say that, “specifically, 12 weeks of curcumin supplementation increased nitric-oxide dependent dilation (P=0.03), whereas no change was observed in the placebo group (P=0.7).” These results were confirmation of similar results seen in the pilot trial conducted prior to this clinical. Kristen Marshall, Marketing Coordinator, Verdure Sciences®, said that she was pleased to see additional support of Longvida’s efficacious ability to promote healthy aging. Twelve weeks of Longvida® also reduced oxidative stress suppression of endothelium-dependent dilation, again with no change seen in placebo.1 The researchers then summarize their findings saying that 12 weeks of Longvida, at 2000mg/day, was found to be safe and well tolerated. It improved resistance and conduit artery endothelial function in healthy middle-aged and older adults, and improvements in endothelial function were mediated by an increase in nitric oxide bioavailability and a reduction in vascular oxidative stress. “Taken together, these data suggest that [Longvida®] may be a promising therapeutic option to improve age-related vascular endothelial function in middle-aged and older adults.”1 “Backed with studies by McFarlin et al for exercise recovery (400mg/day), Santos-Parker et al for vascular endothelial health (2000mg/day), and now available with validated batch specific certification as free of banned substances for athletes worldwide, it is no surprise to see increased interest in our Longvida® curcumin in finished products targeting sports nutrition and joint health for demographics of all ages,” said Marshall. In 2016, Researchers with the University of North Texas examined Longvida® supplementation as therapy for exercise-induced muscle damage (EIMD) and delayed onset muscle soreness (DOMS) impact post training and activities of daily living (ADL). The study utilized an easily managed, once daily 400mg dose of Longvida, versus equivalent placebo, with EIMD from participation in a leg press exercise. This exercise design was chosen to ensure that inflammatory biomarkers presented were appropriately measured and not inflated due to impact to other joints and areas, as can often present in a downhill running model.2 The researchers’ findings, “support the use of oral curcumin supplementation [as Longvida® Optimized Curcumin] to reduce the symptoms of EIMD,” as key biomarkers indicated that supplementation with Longvida® showed statistically significant improvements in inflammatory cytokines (see heat map below).2 Further excitement for Verdure takes form of attendance at Vitafoods in Geneva where Longvida®, Pomella® and WokVel®, will be highlighted at stand H2O which is hosted by distributor Gee Lawson.† References: ¹ Santos-Parker JR et al. Curcumin [Longvida®] supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017 Jan 3. Vol 9(No 1): 187-208. ² McFarlin BK et al. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin [Longvida®]. BBS Clinical. 2016 Feb 18. 5: 72-78. DOI: 10.1016/j.bbacli.2016.02.003 † These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease.