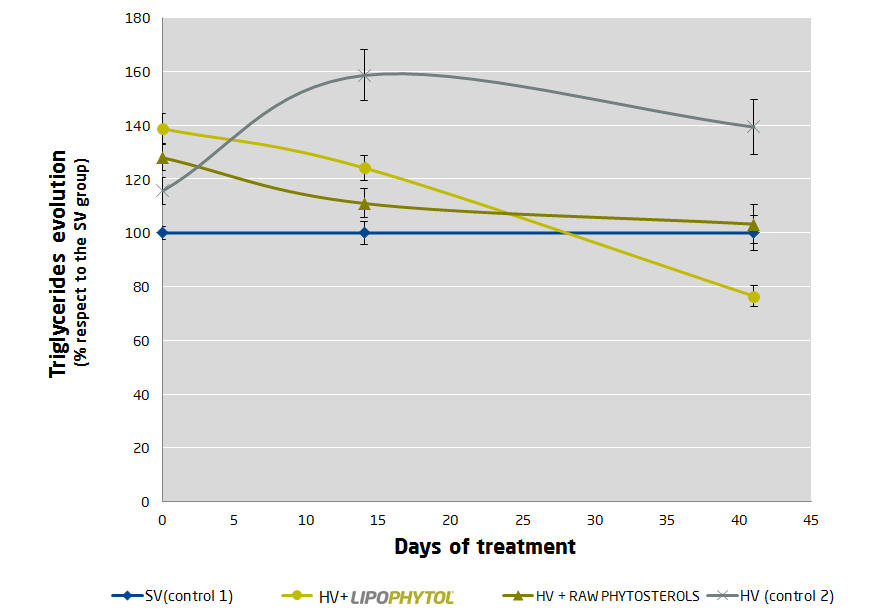
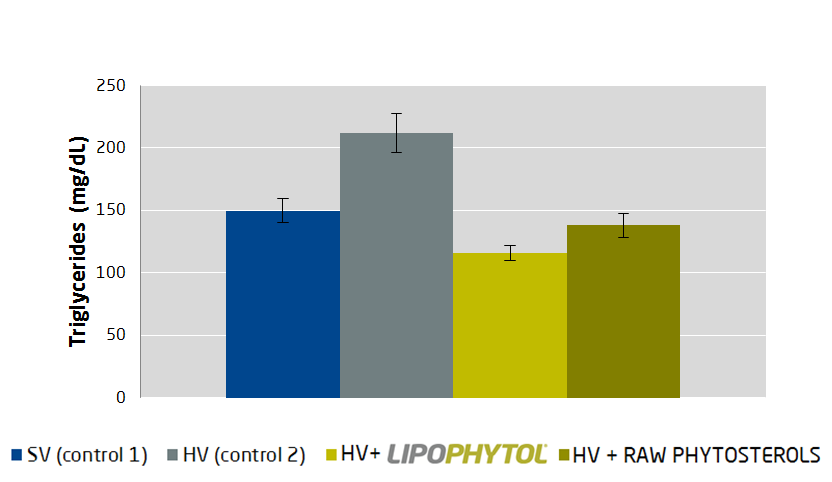
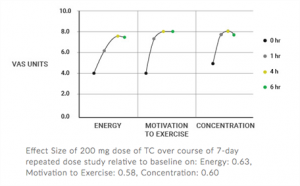
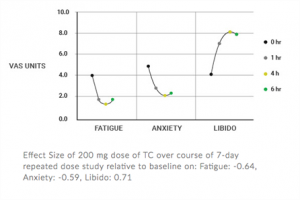
Quatrefolic and Adomet – Depression Focus
DESIO, Italy – Low folate concentrations have been associated with both an increased incidence of depression and an inadequate response to treatment. Folate deficiency is common among depressed people, especially those who don’t respond to antidepressants: major depressive disorder (MDD) currently ranks as the fourth leading disease burden worldwide and is expected to become the second global disease burden in 2020. (Hyman 2006 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3036555/#B1)
Depression is among the top public health concerns worldwide, causing significant disability and disease burden (Whiteford Lancet 2013). Only just in the United States, a total of 200 billion is spent annually on depression, 2 eclipsing the totals spent on cancer and diabetes. In 2012, it was estimated that 16 million people were living with depression in the United States. (1)
Estimates suggest that as many as 12% of men and 25% of women will experience a clinically significant depressive episode in their lives. Of those being treated, approximately 50% will experience a clinically meaningful response to treatment and even fewer will achieve remission (ie, absence of symptoms and return to normal functioning), despite the availability and wide use of several pharmacologic agents. (2)
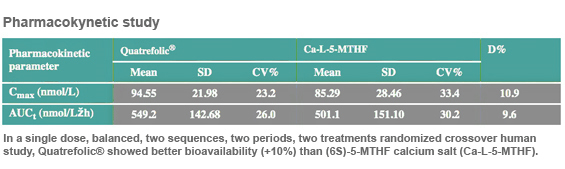
The metabolically active form of folate Quatrefolic®, (6S)-5-MTHF or L-methylfolate, sustains brain functions and the production of neurotransmitters through its role in one carbon metabolism, network of interrelated biochemical reactions that involves the transfer of one carbon methyl groups from one compound to another. (3)
When we look at the methylation cycle its clear that optimum methylation is required to produce neurotransmitters like serotonin and dopamine.
In the one carbon metabolism the remethylation of homocysteine produces methionine, the precursor of S-adenosylmethionine (SAMe) that is the primary methyl group donor in the brain.
SAMe has a key role in mood and cognitive functions because increases levels of dopamine and norepinephrine and accelerate the production, uptake, and re-release of serotonin (4). Adomet® is the commercially stable form of SAMe for nutraceutical applications.
Perturbations of one carbon metabolism, due to low levels of 5-MTHF, critically contribute in decreasing the availability of SAMe and increase the levels of circulating homocysteine, a recognized risk factor for cognitive decline and incident dementia. As a matter of fact low SAMe concentrations have been observed in the cerebrospinal fluid of depressed persons. (3)
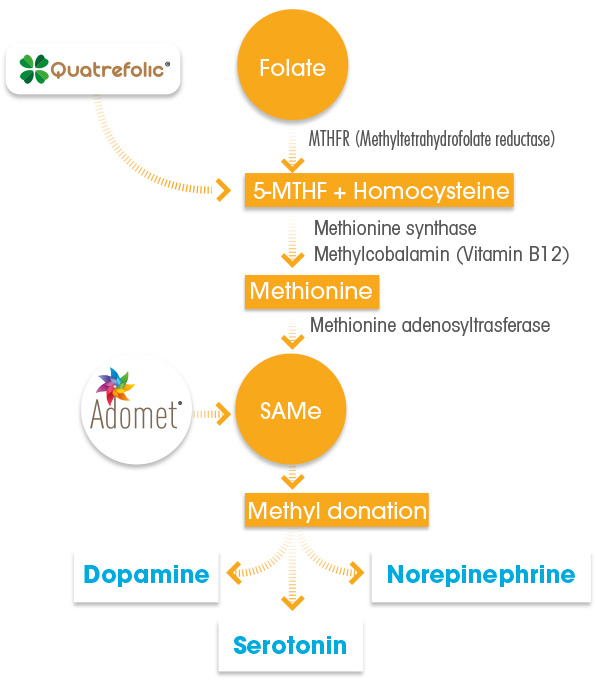
Recent evidence has emphasized how partial- or non-responders to antidepressant drugs may be gravely folate deficient, caused by a polymorphism of the enzyme methylenetetrahydrofolate reductase (MTHFR), which is quite common among patients with depression. These individuals have impaired capacity to convert food folate/folic acid into the metabolic active form, the 5-MTHF or L-methylfolate. Up to 70% of patients with depression test positive for the polymorphism rendering folic acid supplements ineffective for helping in depression.(5)
As a matter of fact, the common approach to raise folate levels by supplementation of the synthetic and man-made folic acid, could be ineffective. Folic acid is the synthetic form of folate and is not biologically active. The body must convert FA into 5-MTHF (L-methylfolate) before the brain can manufacture serotonin, norepinephrine, and dopamine to alleviate depression. People taking FA may still be gravely folate deficient because of the inability of a part of world’s population to assimilate and metabolize folate to 5-MTHF (5,6)
MTHFR polymorphisms are estimated to occur in up to 57% of the population. These subjects need the metabolically active form of folate, the 5-MTHF, the only folate which does not need to be metabolized and is immediately available in the brain to support SAMe release and consequently the production of neurotransmitters. (6,7)
Because patients with low serum folate and hyperhomocysteinemia also have low SAMe levels, it has been suggested that oral supplementation of folate in the form of 5-MTHF (L-Methylfolate) and SAMe may alleviate or prevent depression, reducing also the high levels of homocysteine.
As reported by Papakostas in 2012 supplementation with SAMe, as well as with (6S)-5-MTHF, appears to be efficacious and useful to enhance mood and may be considered an useful adjuvant strategy to increase drug treatment efficacy.
Gnosis’ Quatrefolic®, the patented glucosamine salt of the (6S)-5-MTHF, provides folate in the already biologically active form of 5-methyltetrahydrofolate, without any kind of metabolization. 5-MTHF can reach the systemic circulation and cross the blood-brain barrier, and enter straight the brain cell, for direct utilization in the folate cycle.
Gnosis’ Adomet®, the commercially stable form of SAMe, results from more than 30 years of knowledge of this active and more than 10 patents, ranging from manufacturing processes and stabilization of salts to the production of innovative forms of oral dosage.


 The effectiveness of SAMe Superesse® was evaluated through a symptom scores on a questionnaire, which takes the format of a scale from 0 to 10, or a VAS (visual analogue scale). The questionnaire items used the “Locomotive Syndrome Risk Test”) presented by the Japanese Orthopedic Association as a reference, and the subjective symptoms of the 10 items set were the targets for evaluation.
The effectiveness of SAMe Superesse® was evaluated through a symptom scores on a questionnaire, which takes the format of a scale from 0 to 10, or a VAS (visual analogue scale). The questionnaire items used the “Locomotive Syndrome Risk Test”) presented by the Japanese Orthopedic Association as a reference, and the subjective symptoms of the 10 items set were the targets for evaluation. The new labels better identify Setria® Glutathione as the main ingredient in the antioxidant dietary supplements. Combined with milk thistle extract and Alpha Lipoic Acid™, Setria® provides potent antioxidant protection at the cellular level.* Setria® Glutathione, a premium brand of glutathione – called nature’s “master antioxidant” – replenishes the body’s stores while also protecting cells from oxidative stress and harmful toxins associated with free radicals.*
The new labels better identify Setria® Glutathione as the main ingredient in the antioxidant dietary supplements. Combined with milk thistle extract and Alpha Lipoic Acid™, Setria® provides potent antioxidant protection at the cellular level.* Setria® Glutathione, a premium brand of glutathione – called nature’s “master antioxidant” – replenishes the body’s stores while also protecting cells from oxidative stress and harmful toxins associated with free radicals.*