BLIS M18™ Found to Increase chances of avoiding new Dental Caries
Dunedin, New Zealand and Saint Charles, MO USA – Blis Technologies and Stratum Nutrition announce a new clinical study on the effects of oral-cavity probiotic, BLIS M18™ (Streptococcus salivarius), on the development of dental caries in children. The study was published in Clinical, Cosmetic and Investigational Dentistry (http://dx.doi.org/10.2147/CCIDE.S93066) and represents one of the pioneering attempts to analyze the significance of BLIS M18 in dental practice. The results show that BLIS M18 significantly improves the chances of avoiding new dental caries.
This new randomized, controlled study on BLIS M18™ was conducted on seventy-six children between the ages of 6 and 17 that were classified as high-risk for dental caries on the basis of Cariogram results. Cariogram is a well-recognized, clinically proven software program that looks at different caries-related risk factors to aid clinicians in performing consistent dental caries risk assessments. Thirty-eight subjects (half) were supplemented for 90 days with a formulation containing the oral cavity probiotic BLIS M18 while the other half served as the control group receiving no treatment. The study found that after 90 days of treatment with BLIS M18, there was a statistically significant reduction, by more than 30%, in the global Cariogram outcome, including parameters of “plaque amount” (50% reduction) and “mutans streptococci” (75% reduction). These decreases are likely to be a direct consequence of the BLIS M18 bacteriocins’ interactions with mutans streptococci, as well as M18’s ability to colonize in the oral cavity and produce the beneficial enzymes dextranase and urease which are able to counteract plaque formation and saliva acidity. There was no statistical difference observed in the control group. BLIS M18 also demonstrated a very good safety profile with no treatment-related side effects, as found in previous clinical trials.
Dr. Joseph Evans, Executive Manager of Research & Development for Stratum Nutrition, comments on the study: “Using an objective caries-risk assessment algorithm previously validated in children, young adults and seniors, the investigators have found that dietary supplementation with BLIS M18™ significantly increases the chances of avoiding dental caries in the coming year in children diagnosed as high-risk. Although requiring confirmation in a larger, controlled trial with development of caries as the primary outcome measure, these results suggest that BLIS M18 has the potential to complement a diligent oral hygiene program and nutritionally balanced diet in the ongoing battle against tooth decay.”
The mutans streptococci is one of the contributing factors to the development of dental caries, however its development can be disrupted by the activity of bacteriocins released by the BLIS M18™ probiotic. Different strains of S. salivarius have been shown capable of counteracting the growth of mutans streptococci and of these, the strongest clinical potential has been shown by strain BLIS M18.
Previous trials evaluating BLIS M18™ have revealed its ability to colonize and persist in the human oral cavity, to reduce plaque formation, to lower S. mutans counts in school-aged children, and to modulate the gingival immune response in adults. For more information on these studies, please contact Dr. Joseph Evans atJoseph.Evans@StratumNutrition.com.
Citation: Di Pierro F, Zanvit A, Nobili P, Risso P, Fornaini C. (2015) Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin Cosmet Investig Dent. 7:107-113. http://dx.doi.org/10.2147/CCIDE.S93066
###
About Us: Blis Technologies is the manufacturer of oral probiotic products based on the natural human mouth bacterium Streptococcus salivarius. Stratum Nutrition is the exclusive North American distributor of BLIS probiotic products including BLIS K12 and BLIS M18. BLIS M18™ is a probiotic member of the bacterial species Streptococcus salivarius developed in Dunedin, New Zealand for the support of oral and dental health. Visit http://www.stratumnutrition.com/ingredients/blis-m18/ for more information on BLIS M18.

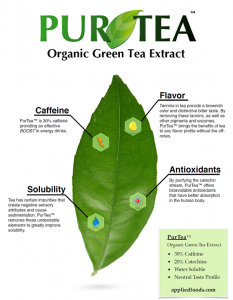
 So why do all this for a commodity like green tea? According to a 2014 report summary by Grandview Research, Inc., notes that functional beverages account for the largest application for antioxidants from tea polyphenols, being more than 40% of total volume sold in 2012. That same summary notes that green tea is the source for 72.5% of those polyphenols (Tea Polyphenols Market Analysis By Application (Functional beverages, Functional food, Dietary supplements) By Product (Green tea, Olong tea, Black tea) And Segment Forecasts To 2020). Since there is a wealth of evidence related to the benefits of green tea compounds, research suggests that using green tea as a natural source of energy might enhance the stimulating effects of caffeine while mitigating negative effects associated with increased oxidation and cardiovascular instability. By decreasing the oxidation reactions taking place during increased energy expenditure green tea extracts can provide an ideal energy source to replace synthetic caffeine. There are some obvious challenges when working with green tea ingredients especially in formulating functional beverages. For Applied Food Sciences, it is about simply making it easier to use and better for you.
So why do all this for a commodity like green tea? According to a 2014 report summary by Grandview Research, Inc., notes that functional beverages account for the largest application for antioxidants from tea polyphenols, being more than 40% of total volume sold in 2012. That same summary notes that green tea is the source for 72.5% of those polyphenols (Tea Polyphenols Market Analysis By Application (Functional beverages, Functional food, Dietary supplements) By Product (Green tea, Olong tea, Black tea) And Segment Forecasts To 2020). Since there is a wealth of evidence related to the benefits of green tea compounds, research suggests that using green tea as a natural source of energy might enhance the stimulating effects of caffeine while mitigating negative effects associated with increased oxidation and cardiovascular instability. By decreasing the oxidation reactions taking place during increased energy expenditure green tea extracts can provide an ideal energy source to replace synthetic caffeine. There are some obvious challenges when working with green tea ingredients especially in formulating functional beverages. For Applied Food Sciences, it is about simply making it easier to use and better for you. Dr. Ajax Mohamed has been named Resident Director, Sabinsa Europe GmbH, the EU division of the Sami-Sabinsa Group of companies. That division just marked its 10th Anniversary with a Sabinsa on Wheels scientific conference.
Dr. Ajax Mohamed has been named Resident Director, Sabinsa Europe GmbH, the EU division of the Sami-Sabinsa Group of companies. That division just marked its 10th Anniversary with a Sabinsa on Wheels scientific conference.


